RESEARCH
Parenting Postpartum (P2)
What is Parenting Postpartum?
Parenting Postpartum (P2) is an extension of Mom Power, an evidence-based and strengths-based program for early mothers. The P2 research program administers Mom Power either virtually or with weekly mailings (control) across 10 weeks. The virtual, 10-group sessions (90 minutes each) deliver weekly content on parenting, self-care, and recovery.
Goals
1
To examine if a virtual delivery of Mom Power will improve drug craving, mood, and parenting stress outcomes for mothers specifically with OUD in the early postpartum.
2
To find out which neurobiological mechanisms are at work through response potential and functional magnetic resonance imaging of the brain.

Context
Every day in the U.S., around 200 people die after opioid overdose, so the opioid epidemic directly impacts millions. With about 20% of all pregnant women prescribed opioids and 2.5% chronically using, the number of pregnant women with OUD more than quadrupled from 1999 to 2014 (1.5 per 1,000 hospital deliveries to 6.5). Although parenting is an essential process for human survival, parenting behaviors are incredibly vulnerable to stress and related psychopathology, including OUD and comorbid depression. Despite the fact that pregnant women with OUD receive “gold standard” buprenorphine treatment (BT) for withdrawal, they remain at high risk for problems through the postpartum, for which treatment is lacking. Mothers with OUD suffer with opioid use relapse, high stress, depression, polysubstance use and maladaptive parenting behaviors that can lead to child maltreatment and costly foster care utilization and contribute to transgenerational cycles of health risk.
Partners: University of Michigan and Stony Brook University
Process Overview
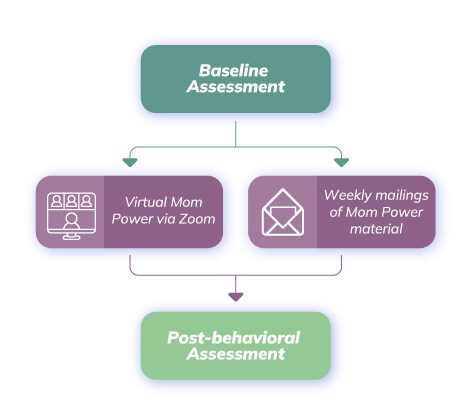
At each site (Stony Brook University and University of Michigan), eligible & consented mothers will undergo baseline assessments (interview/questionnaire surveys and neuroimaging), and then be randomized to virtual Mom Power intervention or control condition.
- The virtual MP intervention consists of 10 weekly zoom sessions with OUD mothers to provide them with Mom Power content.
- The control condition consists of 10 weekly mailings of Mom Power content.
We will conduct a 2-site, 2-group (virtual Mom Power vs. control), pre-post randomized controlled trial of Mom Power for mothers with OUD (n=50, randomized 4:1)
- This consists of delivering a virtual Mom Power group for OUD moms across 10 weeks.
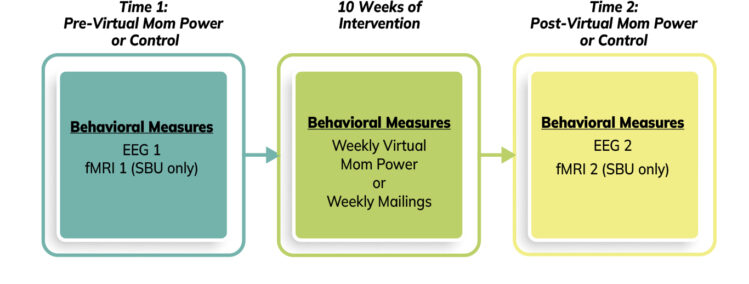
- We will access the outcomes of the program by gathering pre and post fMRI and EEG/ERP data. We will also conduct pre/mid/post maternal self-report data throughout the study.
More Information on Neurobiological Mechanism
To test whether brain mechanisms mediate the clinical benefits of virtual Mom Power for mothers with OUD (H2d), we will:
- Test whether virtual Mom Power caused changes in maternal drug craving, mood & parental stress that are mediated by virtual Mom Power
- Induced changes in maternal AMY and FG responses during own child face mirroring, normalized reciprocal inhibition between HYP and PAG in rs-FC
- Normalized HYP and PAG responses to own baby's cry
Timeline
P2 Study Divisions
- Virtual Mom Power Group: 10 virtual group sessions (90 minutes each); 3 individual virtual support sessions (beginning, midway, and end of group); and weekly between group phone/text check-ins.
- Control - Mom Power Mailings: 10 weekly mailings with content relevant for parenting and the postpartum period; 3 individual virtual support sessions (beginning, midway, and end of mailings); and weekly phone/text check ins.
- ALL groups: Weekly Surveys: Participants complete a short weekly survey while in group or receiving mailings.
Funding Acknowledgment
This project supported by funds from
